Protein is essential for life, playing a crucial role in building and repairing tissues, producing enzymes, and regulating bodily functions. Understanding protein’s importance involves exploring its molecular size, the processes of protein synthesis and catabolism, and how nitrogen balance affects overall health. This post will guide you through these key aspects, highlighting how the structure and solubility of proteins impact their function in the body. Get ready to uncover the vital role protein plays in our everyday lives.
Importance of Protein
- Proteins are the physical basis of life, influencing all functions in living cells.
- Contractile proteins, such as muscle movement, are crucial for motion and locomotion.
- Enzymes, containing proteins, catalyze all biochemical reactions.
- Cell structure and extracellular matrix are largely composed of collagens, the most abundant protein in the human body.
- Proteins like transferrin, Trans membrane proteins, and transcription factors initiate gene transcription.
- Proteins form antibodies, a major component of the immune system.
Molecular Size
- Macromolecules with molecular mass of several thousand or more.
- Polymers made from unbranched chains of amino acids.
- Typical protein contains 200-300 amino acids.
- Some are smaller (peptides) and larger (titin).
- Molecular mass ranges from 6,000 for insulin to several million for some structural proteins.
Protein Synthesis
- Plasma proteins are primarily synthesized in the liver and secreted by the hepatocyte into the circulation.
- Immunoglobulins are synthesized in plasma cells.
- Each protein has a unique amino acid sequence determined by the nucleotide sequence in the specific gene.
- The genetic code is a set of three nucleotides known as codons, each representing a specific amino acid.
- DNA unfolds in the nucleus, with one strand used as a template for mRNA formation.
- The mRNA is then used as a template for protein synthesis by the ribosome.
- The mRNA is manufactured in the cell nucleus and translocated across the nuclear membrane into the cytoplasm.
- mRNA is loaded onto ribosome and read three nucleotides at a time.
- Each codon is matched to its base pairing anticodon on a tRNA molecule.
- The tRNA carries the amino acid corresponding to the recognized codon to the ribosome.
- The process continues until all amino acids form a specific sequence for the polypeptide chain.
- The mRNA code contains initiation and termination codons for the peptide chain.
- Amino acid is activated in a reaction requiring energy and a specific enzyme.
- The tRNA, a short chain of RNA, is attached to the amino acid complex.
- Each amino acid has a specific tRNA with three bases that correspond to the three bases in the mRNA.
- The tRNA carries its specific amino acid to the ribosome and attaches to the mRNA according to the matching codon.
- The preceding amino acid is transferred onto the amino group of the new amino acid, forming a peptide bond.
- The tRNA is released into the cytoplasm, where it can pick up another amino acid.
- The cycle repeats when the terminal codon is reached, releasing the peptide chain and ribosome and mRNA dissociate.
- Protein synthesis occurs at a rate of approximately two to six peptide bonds per second.
- Hormones controlling protein synthesis include thyroxine, growth hormone, insulin, and testosterone, and glucagon and cortisol.

Catabolism and Nitrogen Balance
- Nitrogen has no designated storage depots in the body, unlike fats and carbohydrates.
- Dietary proteins’ biologic value is related to their provision of all necessary amino acids.
- Insufficient dietary quantities can limit the synthesis and lower body levels of essential proteins.
- Proteins are constantly synthesized and degraded, with a balance between protein anabolism (synthesis) and catabolism (breakdown).
- Normal, healthy adults are generally in nitrogen balance, with intake and excretion equal.
- Negative nitrogen balance occurs when more nitrogen is excreted than incorporated into the body, resulting from excessive tissue destruction.
- Protein disintegration occurs in the digestive tract, kidneys, and liver.
- Nitrogen elimination begins intracellularly with protein degradation.
- Two main routes for converting intracellular proteins to free amino acids: a lysosomal pathway and cytosolic pathways.
- Transaminations are central reactions that remove amino acid nitrogen from the body, producing ammonia and ketoacids.
Structure of Protein
Primary Structure
- Represents the number and types of amino acids in the specific amino acid sequence.
- Essential for proper protein function.
- Examples include hemoglobin S formation due to substitution of valine for glutamic acid.
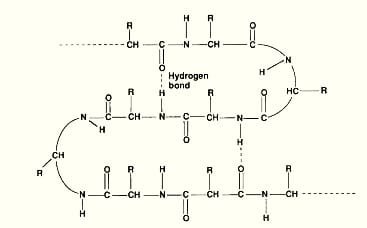
Secondary Structure
- Regularly repeating structures stabilized by hydrogen bonds within the protein.
- Common structures include the α-helix, β-pleated sheet, and turns.
- Adds new properties like strength and flexibility.
Tertiary Structure:
- Refers to the overall shape or conformation of the protein molecule.
- Three-dimensional and results from interaction of side chains.
- Stabilized through hydrophobic effect, ionic attraction, hydrogen bonds, and disulfide bonds.
Quaternary Structure:
- Shape or structure resulting from interaction of more than one protein molecule.
- Holded together by noncovalent forces like hydrogen bonds and electrostatic interactions.
Denaturation:
- Loss of functional and chemical characteristics due to disturbance of secondary, tertiary, or quaternary structure.
Nitrogen Content
Protein Composition and Nitrogen Content
- Proteins consist of carbon, oxygen, hydrogen, nitrogen, and sulfur.
- Nitrogen atoms distinguish proteins from pure carbohydrates and lipids.
- Serum protein’s nitrogen content averages 16%.
- Measurement used for total protein.
Charge and Isoelectric Point P
Protein Charge and Ionizable Groups
- Proteins have ionizable groups on side chains and N- and C-terminal ends.
- Proteins can be positively or negatively charged.
- Acid or basic groups can exist in different charged forms based on pH.
- Lysine, arginine, and histidine side chains have basic groups.
- Glutamate, aspartate, cysteine, and tyrosine side chains have acidic groups.
- pH, pKa, and side chain environment influence charge.
- The Henderson-Hasselbalch equation describes this relationship.

Protein Isoelectric Point (pI) and pH Differences
- As pH increases, acidic and basic groups on proteins deprotonate, converting carboxyl groups to carboxylate anions and ammonium groups to amino groups.
- The pH at which an amino acid or protein has no net charge is known as its isoelectric point (pI).
- A protein with a pH greater than the pI has a net negative charge, while a protein with a pH less than the pI has a net positive charge.
- Proteins vary in their pI values, but most occur in the pH range of 5.5 to 8.
Solubility
- Proteins have a charge on their surfaces, influenced by amino acid number and type and pH.
- Lowest solubility at pI indicates hydrophilic nature.
- Without a charge, protein-protein interactions and precipitation are more likely.
- Blood protein solubility requires pH 7.35 to 7.45.
- Protein separation methods based on solubility are developed.
In conclusion, protein plays a vital role in our body through its importance in protein synthesis, catabolism, and maintaining nitrogen balance. Understanding molecular size and structure helps us see how proteins function and why their solubility matters for health. By focusing on these aspects, we can better appreciate the essential role protein plays in our daily nutrition. Prioritize protein in your diet to support your body effectively and stay healthy. Take action today by making informed choices about the protein sources you include in your meals.
Bibiography
Clinical chemistry: principles, techniques, and correlations/[edited by] Michael L. Bishop, Edward P. Fody, Larry E. Schoeff.—7th ed. CHAPTER 11 n Amino Acids and Proteins 217